Short Answer
|
|
|
1.
|
Use
the terms solvent and solute to describe how to prepare a salt solution.
|
|
|
2.
|
What
is a mixture?
|
|
|
3.
|
Name
two essential roles that enzymes play in cells.
|
|
|
4.
|
What
is mass number?
|
|
|
5.
|
What
does the energy come from that enables you to breathe and think?
|
|
|
6.
|
Where
would you most likely find shared electrons in a water molecule?
|
|
|
7.
|
Distinguish between RNA and DNA.
|
|
|
8.
|
What
are the four groups of organic compounds found in living things?
|
|
|
9.
|
What
accounts for water’s properties of adhesion and cohesion?
|
|
|
10.
|
What
are the main types of chemical bonds?
|
Modified True/False
Indicate
whether the sentence or statement is true or false. If false, change the identified word or
phrase to make the sentence or statement true.
|
|
|
11.
|
Proteins that speed up the rate of chemical reactions in the cell without requiring
high temperatures are antibodies. _________________________
|
|
|
12.
|
The
chemical reaction of 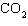 and and 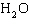 is
irreversible. _________________________ is
irreversible. _________________________
|
|
|
13.
|
When
sugar is dissolved in water, the sugar and water are chemically combined.
_________________________
|
|
|
14.
|
Water
is the greatest solute in the world. _________________________
|
|
|
15.
|
Lipids are important parts of biological membranes and waterproof coverings.
_________________________
|
|
|
16.
|
A
substance made up of only one kind of atom is an element.
_________________________
|
|
|
17.
|
The
symbol for the isotope oxygen-18 is written 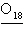 .
_________________________ .
_________________________
|
|
|
18.
|
When
atoms share six electrons, they are joined by a double bond.
_________________________
|
|
|
19.
|
The
substances that are present when a chemical reaction begins are the products.
_________________________
|
|
|
20.
|
The
basic unit of matter is the molecule. _________________________
|
Multiple Choice
Identify the
letter of the choice that best completes the statement or answers the question.
|
|
|
21.
|
Which
of the following terms describes a substance formed by the combination of two or more elements in
definite proportions? a. | compound | b. | isotope | c. | nucleus | d. | enzyme | | |
|
|
|
22.
|
Which
of the following is NOT true about chlorine? a. | It is a poisonous, greenish gas. | b. | It combines with
sodium to form table salt. | c. | It was used to kill many soldiers in World War
I. | d. | It is not
reactive. | | |
|
|
|
23.
|
Amino
acid is to protein as a. | fat is to lipid. | b. | DNA is to
RNA. | c. | sugar is to
fat. | d. | simple sugar is
to starch. | | |
|
|
|
24.
|
Which
of the following illustrates the different types of chemical bonds in order of increasing
attraction? a. | ionic, covalent,
hydrogen | b. | covalent, ionic, hydrogen | c. | hydrogen,
covalent, ionic | d. | hydrogen, ionic, covalent | | |
|
|
|
25.
|
Which
of the following organic compounds is the main source of energy for living things? a. | carbohydrates | b. | lipids | c. | nucleic
acids | d. | proteins | | |
|
|
|
26.
|
A
substance with a pH of 6 is called a. | an acid. | b. | a
base. | c. | both an acid and a base. | d. | neither an acid
nor a base. | | |
|
|
|
27.
|
If an
atom contains 11 protons and 12 neutrons, its atomic number is
|
|
|
28.
|
Democritus believed that atoms a. | were composed of electrons. | b. | were composed of
protons. | c. | could be divided. | d. | could not be
divided. | | |
|
|
|
29.
|
If
the pH of stomach acid and of oven cleaner were measured, a. | both would be
below 7. | b. | both would be above 7. | c. | the pH of
stomach acid would be above 7, but the pH of oven cleaner would be below 7. | d. | the pH of
stomach acid would be below 7, but the pH of oven cleaner would be above 7. | | |
|
|
|
30.
|
What
type of ion forms when an atom loses electrons? a. | neutral | b. | positive | c. | negative | d. | possibly
positive or negative | | |
|
|
|
31.
|
Which
statement is true? a. | Simple sugars are made of
polysaccharides. | b. | Glycerol is made of fatty acids. | c. | RNA molecules
are made of nucleotides. | d. | Amino acids are made of proteins. | | |
|
|
|
32.
|
A
solution is a(an) a. | breaking of a
chemical bond. | b. | chemical reaction. | c. | evenly
distributed mixture of two or more substances. | d. | combination of
two or more liquids. | | |
|
|
|
33.
|
The
most abundant compound in most living things is a. | carbon dioxide. | b. | water. | c. | sodium chloride. | d. | sugar. | | |
|
|
|
34.
|
Which
of the following makes up a molecule of water? a. | one atom of hydrogen and one atom of
oxygen | b. | one atom of sodium and one atom of
chlorine | c. | one atom of hydrogen and two atoms of
oxygen | d. | two atoms of hydrogen and one atom of
oxygen | | |
|
|
|
35.
|
Identify the reactant(s) in the chemical reaction, CO2 + H2O
®
H2CO3. a. | CO2, H2O, and
H2CO3 | b. | CO2 and H2O | c. | H2CO3 | d. | CO2 | | |
|
|
|
36.
|
If
you stir salt into boiling water, you produce a a. | mixture called a suspension. | b. | mixture called a
solution. | c. | solution and suspension. | d. | mixture
only. | | |
|
|
|
37.
|
A
monosaccharide is a a. | carbohydrate. | b. | lipid. | c. | nucleic acid. | d. | protein. | | |
|
|
|
38.
|
What
type of electron is available to form bonds? a. | valence | b. | nucleus | c. | ionic | d. | covalent | | |
|
|
|
39.
|
When
salt is dissolved in water, water is the a. | reactant. | b. | solution. | c. | solute. | d. | solvent. | | |
|
|
|
40.
|
Water
molecules are polar, with the a. | oxygen side being slightly positive and the hydrogen side being
slightly negative. | b. | oxygen and hydrogen sides being slightly
positive. | c. | oxygen and hydrogen sides being slightly
negative. | d. | oxygen side being slightly negative and the hydrogen side being
slightly positive. | | |
|
|
|
41.
|
When
hydrogen and oxygen combine to form water, water is a. | a
product. | b. | a reactant. | c. | both a product
and a reactant. | d. | neither a product nor a reactant. | | |
|
|
|
42.
|
A
substance that accelerates the rate of a chemical reaction is called a(an) a. | catalyst. | b. | lipid. | c. | molecule. | d. | element. | | |
|
|
|
43.
|
A
covalent bond is formed as the result of a. | transferring electrons. | b. | sharing an
electron pair. | c. | transferring protons. | d. | sharing a proton
pair. | | |
|
|
|
44.
|
A map
of eastern North America, showing the pH of rainfall in the various states, indicates that the pH of
rain in New York State varies from 4.22 to 4.40. According to these figures, the most acidic rainfall
in New York State has a pH of a. | 4.22. | b. | 4.30. | c. | 4.35. | d. | 4.40. | | |
|
|
|
45.
|
Ice
floats on water because a. | of cohesion. | b. | ice has a higher
density than water. | c. | water shrinks when it freezes. | d. | water expands
when it freezes. | | |
|
|
|
46.
|
Chemical reactions that release energy a. | will not
occur. | b. | will never explode. | c. | will always
explode. | d. | often occur spontaneously. | | |
|
|
|
47.
|
Which
of the following is NOT a function of proteins? a. | store and transmit heredity | b. | help to fight
disease | c. | control the rate of reactions and regulate cell
processes | d. | build tissues such as bone and muscle | | |
|
|
|
48.
|
Which
of the following statements is true about catalysts? a. | Catalysts slow
down the rate of chemical reactions. | b. | All catalysts are enzymes. | c. | Catalysts are
used up during a chemical reaction. | d. | Catalysts lower the activation energy of a chemical
reaction. | | |
|
|
|
49.
|
What
is the process that changes one set of chemicals into another set of chemicals? a. | cohesion | b. | adhesion | c. | chemical
reaction | d. | dissolving | | |
|
|
|
50.
|
Which
of the following is a use of radioactive isotopes? a. | can determine
the ages of rocks and fossils | b. | can be used to treat cancer and kill bacteria that cause food
to spoil | c. | used as “tracers” to follow the movements of
substances within organisms | d. | all of the above | | |
|